Video
BITCOIN CAINDO SEM PARAR: O QUE ESPERAR DA CRIPTOMOEDA PARA 2026?

ASSINE A FINCLASS COM 40% DE DESCONTO! LIVES DE TIRA DÚVIDAS TODA SEMANA!
https://finc.ly/6258dcf7a5
Nesse vídeo eu mostro por que esse cenário é totalmente possível caso o topo do ciclo já tenha ficado nos 126 mil.
A tese é simples
• Bitcoin sempre operou em ciclos de 4 anos
• Quedas de 75% a 86% já aconteceram antes
• 2026 historicamente seria um ano de baixa
Aqui você vai ver
• O que mudou em cada ciclo do Bitcoin
• Até onde a queda faz mais sentido nos gráficos
• Por que 70k, 60k e até 50k entram no radar
• Como montar uma estratégia sem ansiedade e sem all in
Não é vídeo pra gerar medo.
É vídeo pra você entender risco, preço e estratégia.
Se você acha que BTC só sobe ou que queda é o fim do mundo, esse vídeo vai te incomodar.
Se você investe pensando em 4 anos pra frente, ele vai te ajudar muito.
Assiste até o final e comenta
Em que preço você teria coragem de comprar Bitcoin?
BITCOIN CAINDO SEM PARAR: O QUE ESPERAR DA CRIPTOMOEDA PARA 2026?
00:00 2026 será um bom ano pro BTC?
01:00 Bitcoin aos 60 mil é possível?
02:00 Como funcionam os ciclos de 4 anos do Bitcoin
03:00 Quedas históricas e o padrão dos ciclos passados
04:00 Níveis de preço importantes: 70k e 60k
05:00 Indicadores de longo prazo e média de 200 semanas
06:00 Até onde o Bitcoin pode cair no pior cenário
07:00 Estratégia prática para comprar durante a queda
source
Video
Klartext! Bitcoin: Ich lag falsch!

► Den „BuyTheDip“-Podcast mit Lars Erichsen, Timo Baudzus und mir findet Ihr hier → https://www.youtube.com/@BuyTheDipPodcast oder auf https://buythedip.podigee.io/
► Jetzt gratis sichern 📲 → https://www.hell-investiert.de/app
» Die neue „Hell investiert“-App mit Masterclass, 7 E-Books, exklusiven Analysen, Sprachnachrichten, dem Report, Live-Events & Community – alles an einem Ort & alles 100% kostenlos.
► Hier könnt Ihr meinen Kanal abonnieren → https://www.youtube.com/c/Hellinvestiert?sub_confirmation=1
► Sichere Dir jetzt meinen Report „Telekom – Das sieht super aus!“ – 100% gratis → https://www.hell-investiert.de
In diesem Video spreche ich Klartext über Bitcoin. Mein letzter Einstieg bei 102.000 US-Dollar war rückblickend zu früh, denn der Kurs ist danach noch einmal deutlich gefallen. Genau deshalb nehme ich mir jetzt die Zeit, die Situation offen einzuordnen und meinen aktuellen Plan darzulegen.
Ich erkläre, wie ich mit dem Rücksetzer umgehe, wie meine neue Vorgehensweise aussieht und warum es bei Bitcoin extrem wichtig ist, sauber zwischen langfristigem Investieren und kurzfristigem Handeln zu unterscheiden. Außerdem zeige ich auf, welche Fehler viele Anleger in solchen Phasen machen und wie man sie vermeiden kann, um emotional stabil und strategisch sauber investiert zu bleiben.
► Folge mir jetzt bei LinkedIn → https://www.linkedin.com/in/hellsebastian/
► Ihr findet mich auch auf Instagram → https://www.instagram.com/hell.investiert/
► Die „BuyTheDip“-App! Jetzt anmelden & App downloaden → https://bit.ly/BuyTheDip-free
Inhaltsverzeichnis:
00:00 – Intro & Begrüßung
00:11 – Ihre seid die Besten!
01:26 – Klartext: Mein Bitcoin-Einstieg war zu früh
05:00 – Mein neuer Plan für Bitcoin
08:06 – Was ich tue, wenn ich falsch liege
09:00 – BuyTheDip-Umfrage: So denkt die Community
11:28 – 5 Gründe, die weiter für steigende Kurse sprechen
Copyright Thumbnail:
https://www.freepik.com/premium-psd/golden-bitcoin-cryptocurrency-coins-stacked-isolated-transparent-background_408881975.htm#fromView=search&page=8&position=39&uuid=c0074472-69e9-4c09-bb57-f6fdb7ba10ac&query=bitcoin
https://www.freepik.com/premium-psd/golden-bitcoin-cryptocurrency-coins-stacked-isolated-transparent-background_408881975.htm#fromView=search&page=8&position=39&uuid=c0074472-69e9-4c09-bb57-f6fdb7ba10ac&query=bitcoin
https://www.freepik.com/premium-ai-image/digital-financial-chart_376909021.htm#fromView=search&page=1&position=8&uuid=3d57bf78-06b3-45e9-a219-550a740d8135&query=stock+market+green
https://www.freepik.com/premium-ai-image/green-stock-market-chart_371179575.htm#fromView=search&page=2&position=24&uuid=3d57bf78-06b3-45e9-a219-550a740d8135&query=stock+market+green
Ein wichtiger abschließender Hinweis: Aus rechtlichen Gründen darf ich keine individuelle Einzelberatung geben. Meine geäußerte Meinung stellt keinerlei Aufforderung zum Handeln dar. Sie ist keine Aufforderung zum Kauf oder Verkauf von Wertpapieren. Jeder handelt auf eigene Verantwortung!
Zum Zeitpunkt der Erstellung dieses Beitrags/Videos war der Autor, Sebastian Hell, in folgenden der besprochenen Finanzinstrumente selbst investiert: Bitcoin (siehe Video) | Geplante Änderungen: Keine. Weitere Informationen entnehmen Sie bitte unserem Transparenz-Hinweis zum Umgang mit Interessens-Konflikten → https://www.hell-investiert.de/transparenz-und-rechtshinweise
Ich verwende die Charting-Plattform „TradingView“. Hier kommst Du direkt zu https://de.tradingview.com
Die verwendete Musik wurde unter https://www.audiojungle.net lizensiert | Urheber: MusiCube
► Impressum → http://www.hell-investiert.de/impressum/
#bitcoin
#btc
#krypto
#kryptowährungen
#investieren
#investieren2026
#hellinvestiert
source
Video
I’m Losing Brain Cells | Financial Audit

▶The best budgeting program online, at the most affordable price: https://go.calebhammer.com any questions? Email support@calebhammer.com
▶DITRY MONEY: Get dirty with us 😉 https://shop.calebhammer.com/dirty
▶1-on-1 personal financial help with our licensed/certified coach: https://calebhammer.com/coaching
_______________________
▶Resources I use/would use (with discounts/sign-up bonuses):
1) Checking & Savings: Fee-free overdraft up to $200*, when you set up direct deposit. No credit check. No interest*. No mandatory fees: https://clickurl.ca/caleb-spotme
2) *I’VE MOVED MY INVESTMENTS TO WEBULL* do the same and transfer to my investing app of choice here: https://www.webull.com/k/Caleb and you get: *Cash bonus of $200 – $30,000* depending on initial funding amount, up to 8.1% APY, and up to 3.5% IRA Match.
3) Coursecareers: Get qualified for a better job and increase your income: https://coursecareers.com/a/calebhammer
4) Mint Mobile: Cellphone bills are too expensive, use my link to find a plan starting as low as $15/month in just 15 minutes with my partner MintMobile: https://mintmobile.com/hammer
5) Online security: Protect your online privacy and security NOW and for free by following my link Aura: https://aura.com/hammer
_______________________
▶Our other channels:
1) Vlogs: https://www.youtube.com/@CalebHammerLive/videos
2) Weekend Workplace Podcast: https://www.youtube.com/@WeekendWorkplace/videos
3) Money Makes Cents: https://www.youtube.com/@dollarwiseeducation/videos
_______________________
▶Fun things:
1) Exclusive episodes, private Discord, BTS, and a special thank you: https://www.patreon.com/calebhammer
2) Get your own Hammer Financial Score: https://www.calebhammer.com
3) Merch: The most fun website: https://shop.calebhammer.com
_______________________
▶My socials: https://linktr.ee/calebhammer
▶️Want to be a guest on Financial Audit? We film weekdays in our studio in Austin, Texas (in person only)! To apply, visit: http://calebhammer.com/apply
_______________________
Chapters:
00:00 Job and income
05:30 So they kicked you out…
11:53 You can’t do math?!
16:39 Take care of your stuff
19:00 This is asinine
25:23 I’m losing my mind
29:48 Dirty Money
35:42 They ARE the welfare state
40:30 You’re financing debt
48:40 Listen to your mom!
50:29 Moomoo
58:25 All I’m saying is…
1:02:15 I’m done with this conversation
_______________________
▶Sponsorship and business inquiries: business@calebhammer.com
_______________________
These are some fun words: Caleb Hammer, financial audit, Graham Stephan, Dave Ramsey, Ramsey Show, George Kammel, Rich Dad Poor Dad, Podcast, Budgeting, Investing, Stock Trading, IRA, Roth IRA, 401k, health savings, HSA, 403b, college costs, student loans, debt, snowball, avalanche, pension, retirement, financial independence retire early.
_______________________
▶*Some of the links and other products that appear in this video are from companies for which Caleb Hammer will earn an affiliate commission or referral bonus. Some of the offers mentioned may no longer be available. This is not investment advice.
▶Sponsorship and business inquiries: business@calebhammer.com
source
Video
XRP ETFs CRUSH BTC, ETH & SOL… But XRP Price is STILL DUMPING?! #crypto #cryptocurrency #xrp
Video
URGENT INVESTIGATION: The MOST IMPORTANT Financial Story Ever! #shorts
A 72-hour investigation with potentially massive consequences. We’ve paid a heavy price to bring you the most honest, real-time financial information. This story could change everything. #Investigation #FinancialNews #HonestReporting #RealTimeInfo
Martyn’s Trades on Discord with 1 on 1 support LIVE Stage EVENTS – 90% OFF | Just $2.99
https://discord.com/servers/martyn-lucas-investor-914375589480259635
YouTube Electrum
NEWSLETTER 25% OFF – https://www.sensei.news/25OFF
source
Video
Gold and Silver DUMPED…What about Bitcoin?

Check out ClashPicks: https://www.clashpicks.com/
**Exchange Partners**
🟩Bitunix Exchange ► *$100,000 Deposit Bonus* ► https://bit.ly/3Tmp1Hq
🟦BTCC Exchange ► *10% Deposit Bonus* ► https://bit.ly/4kk20Qa
**Get updates on $Clash (Discord, Giveaway Page, Tournaments)**
🖥️Check Out *Clash’s Website* ► https://georgeplaysclashroyale.com/
💜Join *Clash Discord* ► https://discord.com/invite/georgeplaysclash
🕹️Join *Clash’s Weekly Tournaments* ► https://georgeplaysclashroyale.com/tournament
**Buy $Clash (CA: 6nR8wBnfsmXfcdDr1hovJKjvFQxNSidN6XFyfAFZpump) on**
✅Instructions ► https://georgeplaysclashroyale.com/buy
✅Jupiter Dex ► http://bit.ly/471J57D
✅BTCC Exchange ► https://bit.ly/4kk20Qa
✅MEXC Exchange ► https://bit.ly/42wnMIb
✅Moonshop App ► https://bit.ly/3WbjunI
**Streaming $Clash Play on these Accounts**
📱GeorgePlaysClashRoyale YouTube ► https://www.youtube.com/@Georgeplaysclashroyale
📱GeorgePlaysClashRoyale TikTok ► http://tiktok.com/@georgeplaysclashroyale
🟧CryptoPulse Bootcamp (Learn How to Trade) ►https://cryptosrus.com/bootcamp
🟧CryptoPulse Discord ► https://cryptosrus.com/learn
*Join our free, beginner-friendly Discord group to swap tips, ask questions, and explore the basics of crypto trading, investing, and blockchain. Also chat about portfolios, and off-topic things such as Cars and Legos.*
**Other X Accounts to Follow**
🐤CryptosRus (For News) ► https://x.com/CryptosR_Us
🐤CryptoPulse (For Trades) ► https://x.com/CryptoPulse_CRU
🐤GeorgePlaysClashRoyale (For Clash) ► https://x.com/GeorgePlayClash
🐤ClashPicks (Prediction Market) ► https://x.com/ClashPicks
🔴Full Disclaimer: This video and its contents are for informational purposes only and do not constitute an offer to sell or trade, a solicitation to buy, or recommendation for any security, cryptocurrency, or related product, nor does it constitute an offer to provide investment advice or other related services by CryptosRUs. CryptosRus may have a financial investment with the cryptocurrencies discussed in this video. In preparing this video, no individual financial or investment needs of the viewer have been taken into account nor is any financial or investment advice being offered. Any views expressed in this video were prepared based upon the information available at the time such views were written. Changed or additional information could cause such views to change.
source
Video
The Importance of Financial Education I Robert Kiyosaki

In this video, Robert and Trump talk on both sides of the coin on using DEBT for your Business, and how Financial Education can help you in making that decision.
Disclaimer: This content doesn’t belong me, it is edited and shared only for the purpose of education and Awareness
#advice #debttrap #financialliteracy #financialeducation #investment #kiyosaki #knowledge #richdadpoordad #shorts #trump
Like if you got value from this video and make don’t forget to SUBSCRIBE .
source
Video
Raoul Pal: 10/10 Crash & Bitcoin 2026 Predictions & The AI Trade

In this episode, Nic sits down with Raoul Pal, CEO of Real Vision, to unpack why crypto continues to underperform despite easing global liquidity. While stocks and gold push higher, Bitcoin and altcoins remain weighed down by structural selling, weak risk appetite, and lingering damage from liquidation events.
Raoul explains the key bearish pressures still facing the market, from inventory overhangs and call-selling near resistance to macro uncertainty and fading speculation. With crypto lagging traditional markets, the discussion focuses on whether this is simple consolidation — or a warning of further downside ahead.
~~~~~
🛒 Get The Hottest Crypto Deals 👉 https://www.coinbureau.com/deals/
♣️ Join The Coin Bureau Club 👉 https://hub.coinbureau.com/
📱 Coin Bureau Telegram 👉 https://go.coinbureau.com/yt-telegram
💥 Coin Bureau Discord 👉 https://go.coinbureau.com/cb-discord
📲 Insider Info in our Socials 👉 https://www.coinbureau.com/socials/
👕 Best Crypto Merch 👉 https://store.coinbureau.com
🔥 TOP Crypto TIPS In our Newsletter 👉 https://www.coinbureau.com/newsletters/
💸 Coin Bureau Finance Channel 👉 https://www.youtube.com/@CoinBureauFinance
⭐ More Coin Bureau Channel 👉 https://www.youtube.com/@coinbureaupodcast
📈 Coin Bureau Trading Channel 👉 https://www.youtube.com/@CoinBureauTrading
~~~~~
🔥OUR BRAND PARTNERS🔥
📈Bitget up to 50K USDT Deposit Bonus & GetAgent Plus Trial (Exclusive AI-powered Trading Assistant) 👉 https://go.coinbureau.com/bitget-getagent
📊Join Toobit for 100K USDT Bonus and 50% Lifetime Fee Discount 👉https://www.toobit.pro/t/coinbureau
🔒Get 10% Off Your Tangem Wallet 👉 https://go.coinbureau.com/tangem10
~~~~~
📺 Essential Links 📺
🐥Raoul on X: https://x.com/RaoulGMI
🐥Real Vision on X: https://x.com/RealVision
~~~~~
~ TIMESTAMPS ~
0:00 Intro: Liquidity, rates too high & why Bitcoin should be higher
1:38 Raoul Pal’s 2026 liquidity thesis explained
3:26 Global liquidity, weak dollar & China’s role
3:52 Why Bitcoin “should be around $140K”
7:22 NASDAQ vs global liquidity: the key macro chart
8:18 What really happened during the 10/10 crypto crash
10:51 Why Bitcoin keeps getting suppressed near $100K
12:01 When the selling pressure could finally end
15:25 Quantum computing risk: real threat or FUD?
18:04 Debt risk, corporate Bitcoin treasuries & DATs
21:21 Gold surge explained: lead indicator for Bitcoin
23:10 When gold rotates into crypto
28:34 AI trade: why this is the final technology cycle
39:34 Altcoin outlook: will there be an alt season in 2026?
47:21 Banana Zone thesis: why the real bull run comes later
~~~~~
📜 Disclaimer 📜
The information contained herein is for informational purposes only. Nothing herein shall be construed to be financial legal or tax advice. The content of this video is solely the opinions of the speaker who is not a licensed financial advisor or registered investment advisor. Trading cryptocurrencies poses considerable risk of loss. The speaker does not guarantee any particular outcome.
#bitcoin #crypto #bullmarket #bitcoincrash
source
Video
Financial Audit’s Most Evil Guest

▶ *MAJOR ANNOUNCEMENT* I’m super excited to be launching my MUCH ASKED FOR real estate program (low launch price until end of month) https://calebhammer.com/realestate *OR* to sweeten the deal, my 4-class bundle is now nearly *40% off* !!!!!! Check it out here: https://calebhammer.com/classpack/
▶This relationship is *toxic* … he ameks her cry all the time and she calls him out for yelling at her- watch the post-show here: https://www.youtube.com/channel/UCLe_q9axMaeTbjN0hy1Z9xA/join
▶ Download my budgeting app today: https://calebhammer.com/app – don’t overcomplicate this crap! All you need is an automated and SIMPLE budget. This comes with live CFP sessions, weekly classes, an online community, automatic account connections, my budget-friendly cookbooks, and exclusive discounts on my products Check it out 🙂
Use Yrefy to refinance your private student loans today at: yrefy.com/hammer or call (888) Yrefy-78
Get a 60-day free trial at https://www.shipstation.com/caleb. Thanks to ShipStation for sponsoring the show!
▶▶▶ Download my budgeting app today: https://calebhammer.com/app
▶ Watch this episode’s *POST* *SHOW* + get *MORE* Financial Audit here: https://www.youtube.com/channel/UCLe_q9axMaeTbjN0hy1Z9xA/join
▶EDUCATION: Bundle is 33% off and all courses are 20% off through March 3rd!
1) Bundle my budgeting, debt + investing program for *15%* *off* : https://calebhammer.com/classpack/
2) The best budgeting program online: https://calebhammer.com/budget
3) Get my investing class and I’ll give you a $100 moomoo cash reward: https://calebhammer.com/investing
4) Win with GOOD debt and get out of BAD debt correctly, learn in my debt program: https://calebhammer.com/formula
5) Get your own free Hammer Financial Score: https://www.calebhammer.com
_______________________
▶RESOURCES
1) Checking & Savings: Get up to $500^ before payday when you sign up and set up direct deposit. No credit check. No interest*. No mandatory fees: https://clickurl.ca/caleb-mypay
2) Click this link https://j.moomoo.com/Caleb to get up to 30 free stocks from moomoo U.S when you make a qualified deposit + earn 8.1% on uninvested cash for a limited time for new users!! Terms & Conditions Apply
Securities are offered through Moomoo Financial Inc., Member FINRA/SIPC. The creator is a paid influencer and is not affiliated with MFI and their experiences may not be representative of other moomoo users. Investing is risky. Visit moomoo.com/us/support/topic4_222 for information on the Cash Sweep Program.
3) Get $20 from Acorns for free: sign up to get your bonus https://acorns.com/caleb
4) CourseCareers: Land a high-paying job with no experience or degree by going through an affordable online course: https://coursecareers.com/a/calebhammer
5) The credit building debit card: First 100,000 people to sign up for Fizz with code: HAMMER10 get $10: https://www.joinfizz.com/caleb (paid ad)
6) Advising: Book your free strategy session https://dmnmny.co/caleb to work with Adrianna or any of the other CFPs at Domain Money today!
7) Helium Mobile: save a ton on your phone bill, sign up and get a FREE plan when using promo code CALEB https://hellohelium.com/
8) Online security: Protect your online privacy and security NOW and for free by following my link Aura: https://aura.com/hammer
9) Therapy: Make SonderMind your mental health home in 2025. Sign up at: https://pages.sondermind.com/caleb/
10) Build you own stan store: https://www.stan.store/?ref=CalebHammer
_______________________
▶OTHER CHANNELS:
1) Your Week In Money: https://www.youtube.com/@CalebHammerLive
2) Financial Audit Follow-Ups: https://www.youtube.com/@calebhammerclips
_______________________
Chapters:
00:00 Intro
01:03 Jobs & Income
07:00 let the show begin..
11:50 bro does NOT care
17:45 totally not the price is right haha..
26:41 absolutely embarrassing
32:31 BS amazon spending
37:32 the audacity of this guy.
45:10 and he’s still going.
56:53 asking the real questions here
01:03:51 when you thought it couldn’t get worse lol
01:11:47 how long?
01:20:27 every married man knows that stare
01:27:34 Budget Time!!
01:33:34 Hammer Financial Score
_______________________
▶EXTRA
1) My website: https://calebhammer.com
2) My socials: https://stan.store/calebhammer
3) Want to be a guest on Financial Audit? We film weekdays in our studio in Austin, Texas (in person only)! To apply, visit: http://calebhammer.com/apply
Any questions? Email casting@calebhammer.com
_______________________
▶*Some of the links and other products that appear in this video are from companies for which Caleb Hammer will earn an affiliate commission or referral bonus. Some of the offers mentioned may no longer be available. This is not investment advice.
▶Sponsorship and business inquiries: business@calebhammer.com
source
Video
Bitcoin’s WORST Enemy? [Why Metals Are Winning Now]
![Bitcoin's WORST Enemy? [Why Metals Are Winning Now]](https://wordupnews.com/wp-content/uploads/2026/02/1770273652_maxresdefault.jpg)
Silver hit $117 and Gold breached $5,500—all-time highs. Meanwhile, Bitcoin crashed 30% from its October peak. Is “Digital Gold” dead, or is this the greatest bear trap in crypto history? We break down why Bitcoin ETFs lost $6 billion while Gold ETFs gained $89 billion, the Greenland geopolitical crisis driving the divergence, and the industrial demand powering silver’s rally.
History shows metals pump first, Bitcoin second, altcoins last. With Fed rate cuts expected in Q2 2026 and regulatory clarity coming, this lag could set up Bitcoin’s next explosive move. Strategy accumulated $20.5 billion in BTC during the “weakness.” Are you buying the dip or rotating to metals?
~~~~~
🛒 Get The Hottest Crypto Deals 👉 https://www.coinbureau.com/deals/
♣️ Join The Coin Bureau Club 👉 https://hub.coinbureau.com/
📱 Coin Bureau Telegram 👉 https://go.coinbureau.com/yt-telegram
💥 Coin Bureau Discord 👉 https://go.coinbureau.com/cb-discord
📲 Insider Info in our Socials 👉 https://www.coinbureau.com/socials/
👕 Best Crypto Merch 👉 https://store.coinbureau.com
🔥 TOP Crypto TIPS In our Newsletter 👉 https://www.coinbureau.com/newsletters/
💸 Coin Bureau Finance Channel 👉 https://www.youtube.com/@CoinBureauFinance
⭐ More Coin Bureau Channel 👉 https://www.youtube.com/@coinbureaupodcast
📈 Coin Bureau Trading Channel 👉 https://www.youtube.com/@CoinBureauTrading
~~~~~
🔥OUR BRAND PARTNERS🔥
📈Bitget up to 50K USDT Deposit Bonus & GetAgent Plus Trial (Exclusive AI-powered Trading Assistant) 👉 https://go.coinbureau.com/bitget-getagent
📊Join Toobit for 100K USDT Bonus and 50% Lifetime Fee Discount 👉https://www.toobit.pro/t/coinbureau
🔒Get 10% Off Your Tangem Wallet 👉 https://go.coinbureau.com/tangem10
~~~~~
~ TIMESTAMPS ~
0:00 Intro – Gold & Bitcoin Outperforming Bitcoin
1:40 What’s the Raw Data Saying About Metals’ Outperformance?
3:45 Bitcoin ETF Outflows Forcing The Price Down
4:40 Is Bitcoin Actually a Safe Haven?
5:35 Silver’s Industrial Demand & Role in Green Energy
7:18 Should You Sell Your Bitcoin & Buy Metals?
8:50 Is a Massive Bitcoin Breakout Waiting For Q2?
9:43 Strategy HODLing Strong
10:36 What Closes the Gap Between Gold & Bitcoin?
~~~~~
📜 Disclaimer 📜
The information contained herein is for informational purposes only. Nothing herein shall be construed to be financial legal or tax advice. The content of this video is solely the opinions of the speaker who is not a licensed financial advisor or registered investment advisor. Trading cryptocurrencies poses considerable risk of loss. The speaker does not guarantee any particular outcome.
#bitcoin #gold #silver
source
Video
Gold prices have dropped: Analysts on whether to skip or buy the dip

The gold and silver sell-off continued into Monday, but has steadied somewhat.
We speak to experts about volatility in the metal trade and how investors should play it.
#youtube #gold #silver #investing
About Yahoo Finance:
Yahoo Finance provides free stock ticker data, up-to-date news, portfolio management resources, comprehensive market data, advanced tools, and more information to help you manage your financial life.
– Get the latest news and data at finance.yahoo.com
– Download the Yahoo Finance app on Apple (https://apple.co/3Rten0R) or Android (https://bit.ly/3t8UnXO)
– Follow Yahoo Finance on social:
X: http://twitter.com/YahooFinance
Instagram: https://www.instagram.com/yahoofinance/?hl=en
TikTok: https://www.tiktok.com/@yahoofinance?lang=en
Facebook: https://www.facebook.com/yahoofinance/
LinkedIn: https://www.linkedin.com/company/yahoo-finance
source
-
 Crypto World6 days ago
Crypto World6 days agoSmart energy pays enters the US market, targeting scalable financial infrastructure
-
Crypto World6 days ago
Software stocks enter bear market on AI disruption fear with ServiceNow plunging 10%
-

 Politics6 days ago
Politics6 days agoWhy is the NHS registering babies as ‘theybies’?
-
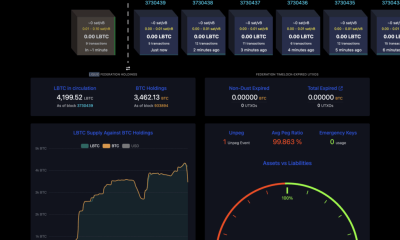
 Crypto World6 days ago
Crypto World6 days agoAdam Back says Liquid BTC is collateralized after dashboard problem
-

 Video2 days ago
Video2 days agoWhen Money Enters #motivation #mindset #selfimprovement
-

 Tech23 hours ago
Tech23 hours agoWikipedia volunteers spent years cataloging AI tells. Now there’s a plugin to avoid them.
-

 Fashion6 days ago
Fashion6 days agoWeekend Open Thread – Corporette.com
-

 NewsBeat6 days ago
NewsBeat6 days agoDonald Trump Criticises Keir Starmer Over China Discussions
-

 Politics3 days ago
Politics3 days agoSky News Presenter Criticises Lord Mandelson As Greedy And Duplicitous
-

 Crypto World5 days ago
Crypto World5 days agoU.S. government enters partial shutdown, here’s how it impacts bitcoin and ether
-

 Sports4 days ago
Sports4 days agoSinner battles Australian Open heat to enter last 16, injured Osaka pulls out
-
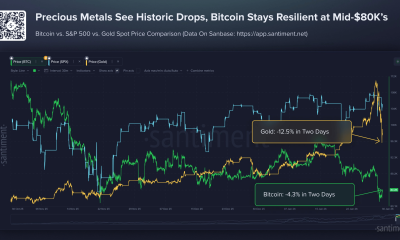
 Crypto World5 days ago
Crypto World5 days agoBitcoin Drops Below $80K, But New Buyers are Entering the Market
-
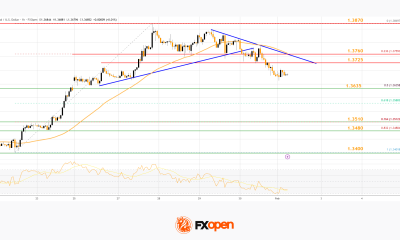
 Crypto World3 days ago
Crypto World3 days agoMarket Analysis: GBP/USD Retreats From Highs As EUR/GBP Enters Holding Pattern
-

 Crypto World5 days ago
Crypto World5 days agoKuCoin CEO on MiCA, Europe entering new era of compliance
-
Business5 days ago
Entergy declares quarterly dividend of $0.64 per share
-

 Sports3 days ago
Sports3 days agoShannon Birchard enters Canadian curling history with sixth Scotties title
-

 NewsBeat2 days ago
NewsBeat2 days agoUS-brokered Russia-Ukraine talks are resuming this week
-

 NewsBeat3 days ago
NewsBeat3 days agoGAME to close all standalone stores in the UK after it enters administration
-

 Crypto World1 day ago
Crypto World1 day agoRussia’s Largest Bitcoin Miner BitRiver Enters Bankruptcy Proceedings: Report
-

 Crypto World6 days ago
Crypto World6 days agoWhy AI Agents Will Replace DeFi Dashboards








