When we think about iron imbalance, most people are familiar with iron deficiency and the health problems it can cause. What many may not realise is that the opposite problem, iron overload, can be just as serious – yet many aren’t even aware of the condition.
Haemochromatosis is an inherited genetic condition that affects the amount of iron the body absorbs. The condition disproportionately affects people of Irish, Scottish and Welsh descent, which is why it’s been nicknamed the “Celtic curse”. Yet this label can be misleading as it is also prevalent in other northern European countries – and this may contribute to why so many cases still continue to go undiagnosed today.
Haemochromatosis causes the body to absorb too much iron from food. Iron is essential for health, particularly for the oxygen-carrying capacity of red blood cells.
In healthy people, this iron is safely stored in the bone marrow and liver in the form of a protein called ferritin. But in people with haemochromatosis, iron stores gradually exceed safe limits. As the body has no effective way to excrete this excess iron, this means the mineral accumulates in their tissues and organs, resulting in damage to these tissues and organs.
Most cases of haemochromatosis are caused by mutations in a gene called HFE, which regulates hepcidin – a hormone made by the liver that helps regulate iron absorption. When this system is disrupted, iron absorption continues unchecked. In people with haemochromatosis, this mutation is usually inherited from both parents.
Iron overload can also occur in people who need repeated blood transfusions – such as those with sick cell disease.
The liver is particularly vulnerable, and excess iron can cause inflammation, irreversible scarring and damage and, in advanced cases, liver cancer. Once liver storage capacity is exceeded, iron begins to accumulate in other organs – including the pancreas, heart, joints and brain, impairing their function.
Diagnosing iron overload
Genetic haemochromatosis has been identified from human remains as far back as bronze age and neolithic Irish populations. It’s thought that humans with the HFE mutation were resistant to iron deficiency caused by iron-poor diets, offering a survival advantage.
But while haemochromatosis is more common people of Celtic ancestry, the condition can affect anyone. The “Celtic curse” label may even have unintentionally contributed to the misconception that the condition is rare.
In reality, around one in 200 people of northern European ancestry carry the mutations that can cause the condition. However, in Ireland, around one in 83 people carry this mutation.
Before the genetic basis of haemochromatosis was discovered, patients were often diagnosed incidentally or after developing cirrhosis or diabetes.
Today, it’s widely understood that symptoms can be wide ranging and of varying severity. The most common complaint is joint pain, particularly affecting the knuckles and hands. Other symptoms include chronic fatigue, bronzing of the skin, reduced libido, heart rhythm problems and difficulties with memory or concentration.
24K-Production/ Shutterstock
Many people have no obvious symptoms for decades – and when symptoms do occur, they’re often attributed to age-related conditions, such as arthritis. This makes haemochromatosis easy to miss.
Large UK population studies suggest that up to 40% of patients with genetic haemochromatosis will develop at least one symptom related to iron overload in their lifetime. Men with the condition are at the highest risk of developing liver cancer.
Despite this, many people remain undiagnosed – even when serious organ damage has already occurred. According to the charity Haemochromatosis UK , undiagnosed haemochromatosis costs the NHS an estimated £300 million per year, due to to avoidable illness and complications associated with the condition.
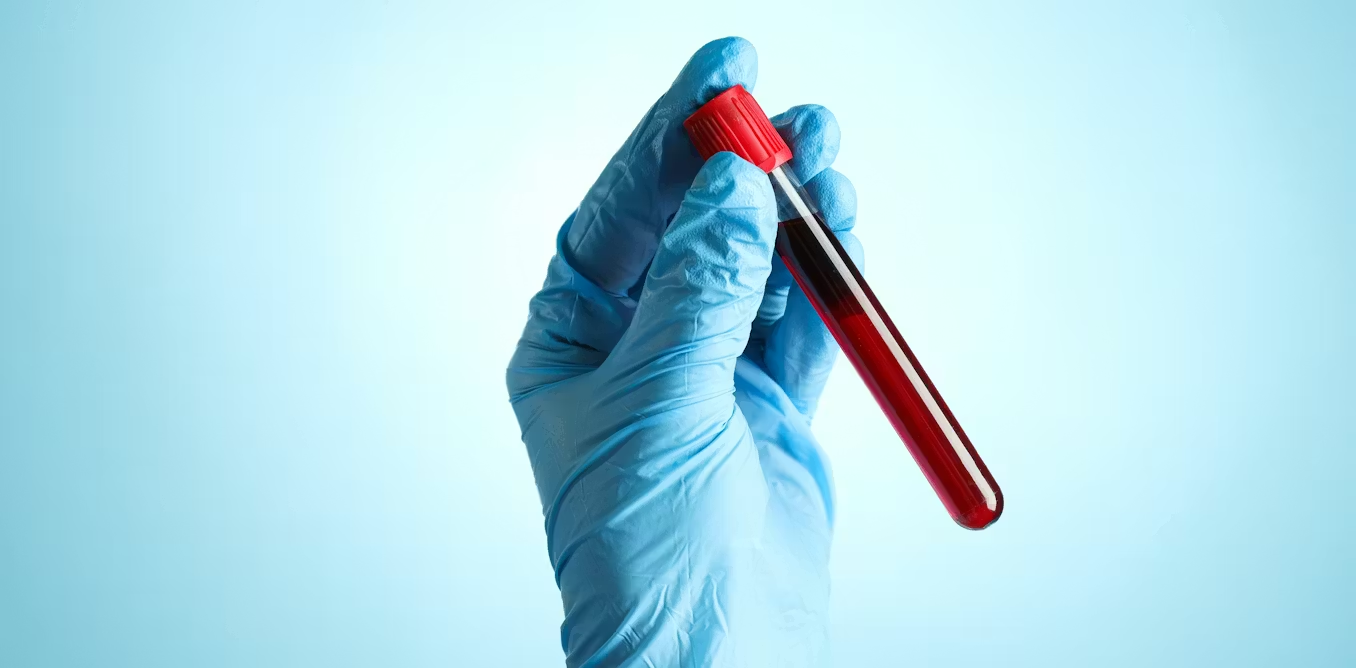
Diagnosing haemochromatosis is actually quite straightforward. Blood tests are performed to measure iron and liver function. If results suggest iron overload, genetic testing can confirm the diagnosis.
But the challenge is knowing who to test. Because symptoms are often vague and the condition is still perceived as rare, haemochromatosis is often overlooked.
Many people are diagnosed incidentally during routine blood tests for unrelated reasons. When a diagnosis is made, close relatives are usually offered testing because of the inherited nature of the condition.
When diagnosed, treatment is simple and highly effective. The standard approach is regular blood removal, known as venesection or phlebotomy. Removing blood removes mobilises stored iron to make new blood cells, helping to deplete these stores. Initially this may be weekly or fortnightly until iron levels normalise – followed by lifelong monitoring and occasional maintenance venesections.
Dietary changes, such as avoiding iron and vitamin C supplements, limiting alcohol and reducing red meat consumption, may help slow iron accumulation, but these cannot replace or be as effective as venesection.
Not all patients tolerate regular blood removal – particularly older people or those whose veins may not be as visible or easy to access. In these cases, iron-chelating drugs (which make it possible for iron to be passed in urine) can be used. However, their side-effects – such as diarrhoea, pain and tiredness – limit their use.
Encouragingly, new treatments are emerging. Drugs that mimic hepcidin, restoring the body’s natural iron regulation, are currently being trialled. Other approaches aim to block ferroportin (which transports iron), using a synthetic inhibitor that competes against the natural protein and is known to reduce iron levels in mice.
Haemochromatosis is common, treatable, and when caught early, the health effects are largely preventable. The greatest challenge is increasing awareness among both doctors and the public, so the condition can be caught before it causes serious harm.