Up to US$600 million of shares to be repurchased pursuant to amended normal course issuer bid
US$605 million return of capital and share consolidation expected to be completed in May
TORONTO, Feb. 25, 2026 /PRNewswire/ — Thomson Reuters (TSX/Nasdaq: TRI) today announced that it plans to repurchase up to US$600 million of its common shares under an amended normal course issuer bid (NCIB) that has been approved by the Toronto Stock Exchange (TSX) and that it plans to return US$605 million to shareholders through a return of capital transaction.
Amended Normal Course Issuer Bid
Shares will be repurchased for the new US$600 million repurchase program under an amended NCIB. The amended NCIB, which has been accepted by the TSX, will become effective on February 27, 2026. The amended NCIB will increase the maximum number of common shares that may be repurchased by an additional 6 million. Under the amended NCIB, up to 16 million common shares (representing approximately 3.55% of the company’s 450,687,724 issued and outstanding shares as of August 12, 2025) may be repurchased between August 19, 2025 (the Effective Date) and August 18, 2026. The NCIB, as originally approved in August 2025, contemplated the repurchase of up to 10 million common shares. To date under the current NCIB, Thomson Reuters has repurchased 6,022,437 common shares for a total cost of approximately US$1.0 billion, representing an average price of US$166.05 per share.
Under the amended NCIB, shares may be repurchased on the TSX, the Nasdaq Global Select Market (Nasdaq) and/or other exchanges and alternative trading systems or by such other means as may be permitted by the TSX and/or the Nasdaq or under applicable law. Based on the average daily trading volume on the TSX of 364,105 for the six months preceding the Effective Date (net of repurchases made by TR during that time period), daily purchases are limited to 91,026 common shares, other than block purchase exceptions. Any shares that are repurchased will be cancelled.
Prior to its next regularly scheduled quarterly blackout period, Thomson Reuters intends to enter into an automatic share purchase plan (ASPP) with its broker to allow for the purchase of shares under the NCIB during pre-determined times when the company would ordinarily not be permitted to purchase shares due to customary blackout periods or other regulatory restrictions. Purchases under the ASPP are made by the company’s broker based upon parameters set by Thomson Reuters when it is not in possession of material non-public information relating to the company or the shares. The ASPP will be entered into in accordance with the requirements of the TSX and applicable Canadian and U.S. securities laws, including Rule 10b5- 1 under the U.S. Exchange Act of 1934, and will terminate when the NCIB expires, unless terminated earlier in accordance with its terms. All purchases made under the ASPP are included in computing the number of shares purchased under the NCIB. Outside of pre-determined blackout periods, shares may be purchased under the NCIB based on management’s discretion, in compliance with TSX rules and applicable securities laws.
Decisions regarding any future share repurchases will depend on certain factors, such as market conditions, share price and other opportunities to invest capital for growth. Thomson Reuters may elect to suspend or discontinue share repurchases at any time, in accordance with applicable laws.
Return of Capital
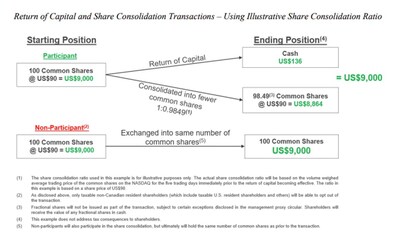
Thomson Reuters will return gross proceeds derived from the May 2024 sales of London Stock Exchange Group shares through a return of capital consisting of a special cash distribution of US$605 million in the aggregate, or approximately US$1.36 in cash per participating share (estimated based on the number of common shares issued and outstanding as of February 24, 2026 and assuming no shareholders opt-out of the return of capital transaction), followed by a share consolidation, or “reverse stock split”, which will reduce the number of common shares on a basis that is proportional to the special cash distribution. To that end, the share consolidation ratio will be based on the volume weighed average trading price of the common shares on the Nasdaq Stock Market LLC for the five trading days immediately prior to the transactions becoming effective.
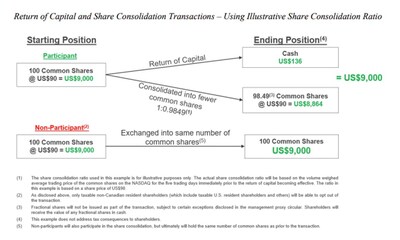
The proposed return of capital is intended to distribute cash on a basis that is generally expected to be tax-free for Canadian tax purposes. Taxable non-Canadian resident shareholders (which include taxable U.S. resident shareholders and others) will be able to opt out of the return of capital. This right to opt out is being provided to those shareholders because in jurisdictions other than Canada the tax consequences of not participating in the return of capital may be preferable to those associated with participating in the return of capital. A taxable non-Canadian resident shareholder that chooses to opt out will not receive the special cash distribution and will continue to hold the same number of Thomson Reuters shares that they currently hold. Taxable non-Canadian resident shareholders are strongly urged to read the management proxy circular and other related materials carefully and to consult with their financial, tax and legal advisors prior to making any decision with respect to the return of capital and share consolidation transactions.
Shareholders will be asked to approve the proposed return of capital and share consolidation transactions at a special meeting of shareholders of Thomson Reuters to be held on Tuesday, April 28, 2026 at 12:00 p.m. (Toronto time). The proposed transactions require approval by at least two-thirds of the votes cast at the shareholder meeting. The board of directors of the company is unanimously recommending that shareholders vote in favor. Woodbridge has indicated that it plans to do so and, accordingly, it is expected that the shareholder vote will pass. The proposed transactions also require the approval of the Ontario Superior Court of Justice (Commercial List). If shareholder and court approval are obtained, Thomson Reuters expects to effect the proposed transactions in early May.
Full details of the proposed return of capital and share consolidation transactions will be described in the company’s management proxy circular and other related materials. Those documents are expected to be mailed or otherwise distributed to shareholders, filed with applicable Canadian securities regulatory authorities and made available without charge on SEDAR+ at www.sedarplus.ca and made available without charge on EDGAR at www.sec.gov, and posted on the company’s website at tr.com, in mid-March.
Thomson Reuters
Thomson Reuters (TSX/Nasdaq: TRI) informs the way forward by bringing together the trusted content and technology that people and organizations need to make the right decisions. The company serves professionals across legal, tax, audit, accounting, compliance, government, and media. Its products combine highly specialized software and insights to empower professionals with the data, intelligence, and solutions needed to make informed decisions, and to help institutions in their pursuit of justice, truth and transparency. Reuters, part of Thomson Reuters, is a world leading provider of trusted journalism and news. For more information, visit tr.com.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
Certain statements in this news release are forward-looking statements within the meaning of Canadian and U.S. securities laws, including statements relating to the company’s plans to repurchase up to US$600 million of its common shares; the timing for the approval and implementation of the return of capital and share consolidation transactions, and the filing of materials related thereto; and the anticipated tax treatment for shareholders participating in the return of capital and share consolidation transactions and those opting out of the return of capital. These forward-looking statements are based on certain assumptions and reflect our company’s current expectations. As a result, forward-looking statements are subject to a number of risks and uncertainties that could cause actual results or events to differ materially from current expectations, including other factors discussed in materials that Thomson Reuters from time to time files with, or furnishes to, the Canadian securities regulatory authorities and the U.S. Securities and Exchange Commission. There is no assurance that the return of capital and share consolidation transactions will be completed or that other events described in any forward-looking statement will materialize. Except as may be required by applicable law, Thomson Reuters disclaims any obligation to update or revise any forward-looking statements.
CONTACTS
 View original content to download multimedia:https://www.prnewswire.com/news-releases/thomson-reuters-announces-new-us600-million-share-repurchase-program-and-us605-million-return-of-capital-and-share-consolidation-transactions-302696958.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/thomson-reuters-announces-new-us600-million-share-repurchase-program-and-us605-million-return-of-capital-and-share-consolidation-transactions-302696958.html
SOURCE Thomson Reuters