Business
Popular weight-loss drugs to come without any ‘suicidal thoughts’ warning
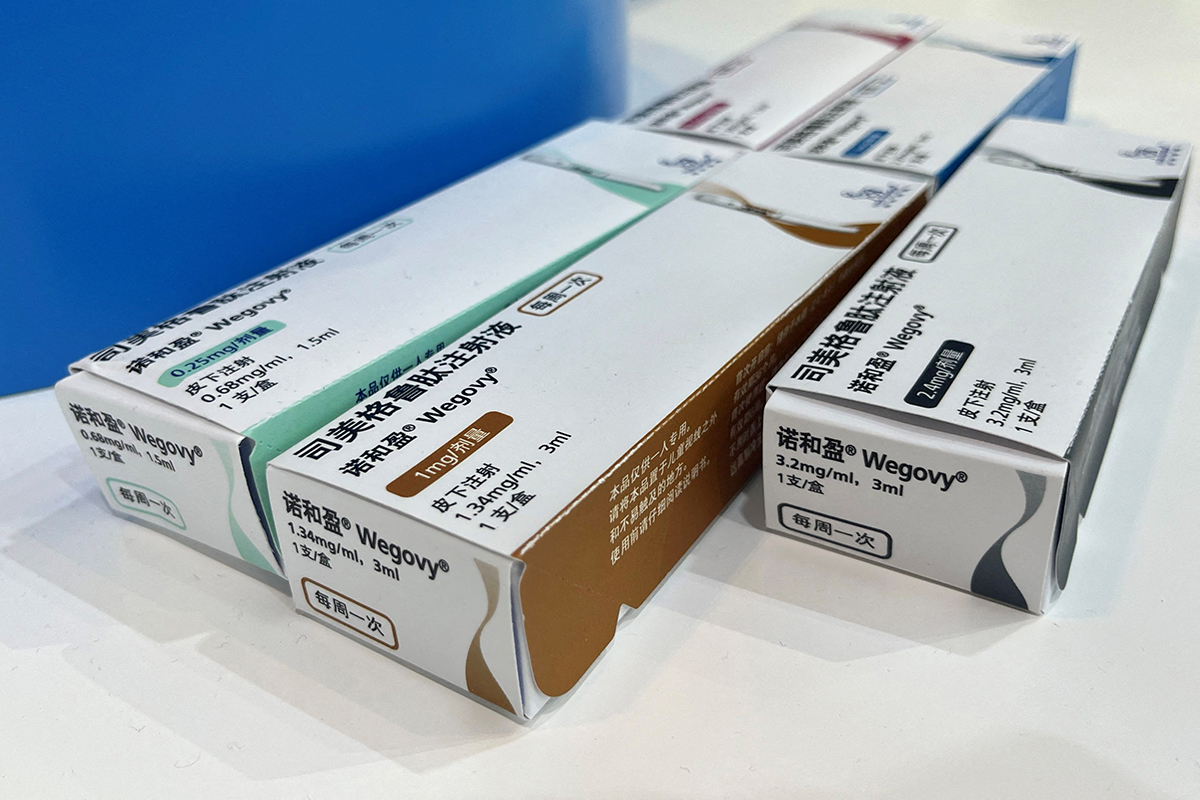
The US federal health regulator, the Food and Drug Administration (FDA), asked drugmakers to remove warnings about a potential risk of suicidal thoughts from widely used GLP-1 weight-loss drugs, including Novo Nordisk’s Wegovy and Eli Lilly’s Zepbound.
This also covers Novo’s older weight-loss drug, Saxenda.
A review by the FDA found no evidence linking GLP-1 receptor agonists to an increased risk of suicidal thoughts or behaviour. The FDA’s move could ease a key safety overhang for the fast-growing class of medicines that are being tested or used for conditions beyond weight loss, including cardiovascular issues, fatty liver disease and sleep apnea.
The FDA said the warnings were part of their original approval, based on reports of such events observed with a variety of older medicines used or studied for weight loss. A Reuters review of the agency’s adverse-event database in 2023 had found that it received 265 reports of suicidal thoughts or behaviour in patients taking these or similar medicines since 2010.
Key GLP-1 drugs reviewed
GLP-1 receptor agonists, originally developed to treat type 2 diabetes, mimic a gut hormone that suppresses appetite, creating a feeling of fullness.
Novo’s Saxenda contains liraglutide and Wegovy contains semaglutide as their respective active ingredients, while Lilly’s Zepbound contains tirzepatide.
Novo’s spokesperson said the company is happy to see the FDA’s recommendation to remove the warning from Saxenda and Wegovy’s labels that currently include risks of suicidal ideation and behaviour.
A Lilly spokesperson said the company appreciates “the FDA’s careful consideration of this important safety issue”, adding Lilly will “continue to work with the FDA on next steps to ensure that appropriate safety information is available to prescribers.”
The labels for these drugs in the European Union do not carry such warnings.
The regulator said on Tuesday it had conducted further analyses of placebo-controlled clinical trials involving GLP-1 drugs, which did not show an increased risk of suicidal thoughts or behaviour versus the placebo, nor other psychiatric side effects such as anxiety, depression, irritability or psychosis.
The review covered 91 trials involving 107,910 patients, including 60,338 who received a GLP-1 drug and 47,572 who received placebo, it said.










