Business
Rain and politics driving up half-term holiday bookings, travel agents say

Business
Politics And The Markets 02/13/26

This is the forum for daily political discussion on Seeking Alpha. A new version is published every market day.
Please don’t leave political comments on other articles or posts on the site.
The comments below are not regulated with the same rigor as the rest of the site, and this is an ‘enter at your own risk’ area as discussion can get very heated. If you can’t stand the heat… you know what they say…
More on Today’s Markets:
Moderation Guidelines:
We remove comments under the following categories:
- Personal attacks on another user account
- Anti-Vaxxer or covid related misinformation
- Stereotyping, prejudiced or racist language about individuals or the topic under discussion.
- Inciting violence messages, encouraging hate groups and political violence.
Regardless of which side of the political divide you find yourself, please be courteous and don’t direct abuse at other users.
For any issue with regards to comments please email us at : moderation@seekingalpha.com.
Seeking Alpha’s Disclosure: Past performance is no guarantee of future results. No recommendation or advice is being given as to whether any investment is suitable for a particular investor. Any views or opinions expressed above may not reflect those of Seeking Alpha as a whole. Seeking Alpha is not a licensed securities dealer, broker or US investment adviser or investment bank. Our analysts are third party authors that include both professional investors and individual investors who may not be licensed or certified by any institute or regulatory body.
Business
FTC warns Apple over alleged lack of conservative news

The tech giant is facing pressure over claims that its news app does not feature articles from conservative outlets.
Business
Ground beef recall: 23,000 pounds over E. coli contamination risk

Check out what’s clicking on FoxBusiness.com.
Federal regulators said nearly 23,000 pounds of raw ground beef are being recalled over potential E. coli contamination.
The U.S. Department of Agriculture (USDA) announced the Class1 recall Wednesday, warning that the product poses a high risk of causing “serious, adverse health consequences or death.”
The affected packages were produced on Jan. 14 by Idaho-based CS Beef Packers and were shipped to distributors in California, Idaho and Oregon.
Officials said the products were intended for further distribution to foodservice locations, such as restaurants and cafeterias, rather than for direct retail sale at grocery stores.

Roughly 23,000 pounds of ground beef were recalled over potential E. Coli contamination. (USDA / Fox News)
As of Wednesday, there have been no confirmed reports of illness associated with the recalled product, USDA said.
The recalled items include 10-pound cylindrical packages, or chubs, of “Beef, Course Ground, 73L,” 10-pound chubs of “Fire River Farms Classic Beef Fine Ground 73L” and 10-pound chubs of “Fire River Farms Classic Beef Fine Ground 81L, with case codes 18601, 19583 and 19563, respectively.
All products have a “Use/Freeze By” date of Feb. 4, 2026, with time stamps between 07:03 and 08:32, printed on two stickers on the outside of the cardboard cases.
All products have a “Use/Freeze By” date of Feb. 4, 2026, with time stamps between 07:03 and 08:32. The date and time stamps appear on the clear packaging of the meat products and on two stickers on the outside of the cardboard cases.
RECALL OF CHEESE PRODUCTS UPGRADED TO HIGHEST DANGER LEVEL OVER LISTERIA-CAUSING BACTERIA: FDA

The U.S. Department of Agriculture recalled nearly 23,000 pounds of ground beef intended for foodservice locations. (USDA / Fox News)
The issue was identified during testing by the department’s Food Safety and Inspection Service (FSIS) at a downstream customer, with results showing the presence of E. coli O145.
Foodservice locations should check their freezers and not serve any of the suspicious products, regulators said, adding that customers should throw them away or return them to the place of purchase.
E. coli O145 infection typically causes diarrhea, often bloody, and vomiting two to eight days after exposure, with an average of three to four days.
Doctors usually diagnose the infection with a stool test. Treatment typically involves vigorous rehydration and other supportive care, and most people recover within a week.
POPULAR SALAD DRESSINGS, SOLD AT COSTCO AND REPORTEDLY PUBLIX, RECALLED OVER ‘FOREIGN OBJECTS’

Ground beef is shown in the Chronicle Studio Tuesday, Jan. 18, 2011, in Houston. (Brett Coomer/Houston Chronicle via Getty Images / Getty Images)
In rare but serious cases, older adults, children under 5 and people with weakened immune systems can develop a type of kidney failure called hemolytic uremic syndrome (HUS). It is marked by easy bruising, paleness and decreased urine output.
GET FOX BUSINESS ON THE GO BY CLICKING HERE
Officials also stressed that consumers should always cook ground beef to an internal temperature of 160 °F to kill harmful bacteria.
Business
Iron Mountain Incorporated 2025 Q4 – Results – Earnings Call Presentation (NYSE:IRM) 2026-02-12

Q4: 2026-02-12 Earnings Summary
EPS of $0.61 beats by $0.02
| Revenue of $1.84B (16.56% Y/Y) beats by $39.87M
Seeking Alpha’s transcripts team is responsible for the development of all of our transcript-related projects. We currently publish thousands of quarterly earnings calls per quarter on our site and are continuing to grow and expand our coverage. The purpose of this profile is to allow us to share with our readers new transcript-related developments. Thanks, SA Transcripts Team
Business
HUL sees demand recovery as rural, urban traction improves; Q3 volumes rise 4%

However, it reported 70 basis point contraction in operating margin before depreciation and amortisation (Ebitda margin) at 23.3% driven by labour code related charges; the margin still remained above the company’s guidance band of 22-23% indicating lack of any stress from operating costs such as raw material prices and inventory management.
The FMCG major expects second half of the current fiscal year ending in March 2026 to be better than the first half and to report even better numbers next year. Its optimism is driven by progress in portfolio and channel transformation, and better macroeconomic scenario including improved consumer sentiments and better consumption demand in rural regions and improving urban traction.
 Agencies
Agenciesgame is on Co is betting on rebound in rural & urban consumption and portfolio transformation
The company’s shares fell 2% on Thursday after it reported a 30% YoY decline in net profit for the December quarter, largely on account of a one-off impact from labour code provisions. Excluding this and one-time gain from sales of the ice cream division, net profit grew by a modest 1%.
HUL’s inorganic growth strategy to expand in new consumer segments is paying off. The acquisition of Minimalist in January last year has helped the company to gain traction in the premium skincare space. The brand has grown faster under HUL. Its sales are not disclosed separately but is included in the beauty & wellbeing division. This division’s revenue grew fastest among all categories, rising 11% year-on-year and 5.3% sequentially. This segment’s share in total revenue has gradually increased to 24.2% in the December 2025 quarter from 20.8% in March 2025.
HUL’s decision to buy the remaining 49% stake in Zywie Ventures (Oziva), appears to be a part of the same strategy to drive long-term growth.
Business
Taylor Swift asks US government to block 'Swift Home' trademark

Her team argued that a bedding firm’s designs showed similarities to her trademarked signature.
Business
Freehills, WA Inc twist in Tronox injunction fight as judge stands aside

Supreme Court judge Gary Cobby stood aside from an injunction battle after a self-styled whistleblower pointed to his work three decades ago with law firm Freehills and its connections to WA Inc.
Business
Rox calls on MACA for gold plant

Rox Resources has engaged MACA to build a processing plant for ore from the Youanmi gold project, as it inches closer to a final investment decision at the historic site.
Business
ETMarkets Smart Talk | Not rock-bottom yet, but India looks attractive vs mid-2024 excesses: Rahul Singh

While the market may not be at “rock-bottom” levels that warrant aggressive allocation, the excess froth seen in pockets such as manufacturing, defence and capital goods has largely receded, bringing the valuation premium over other emerging markets down significantly.
In an interaction with ETMarkets Smart Talk, Rahul Singh, CIO–Equities at Tata Asset Management, explains why India is now better positioned to attract its fair share of emerging market flows, how earnings growth is gradually reviving, why IT may no longer be a drag on profitability, and why investors should look beyond just gold and silver when playing the commodities theme.
He also shares his take on mid- and small-cap opportunities as valuation gaps narrow. Edited Excerpts –
Q) Thanks for taking the time out. It looks like there is some nervousness on D-Street post Budget then it got stabalized. How should investors decode?
A) Foreign Institutional Investors (FIIs) do not have only India to invest in. In mid-2024, India valuations were at an 80–90% premium to other emerging markets (EMs).
After that, we saw earnings growth slowdown and other economies benefited either because of participating in the Artifical Intelligence (AI) theme or because China recovery post stimulus. So global capital followed there.
Now growth is coming back in India and the valuation premium has come down to 50%. It’s still at a premium but much lower than 2024.
We have reached a point where if emerging markets start getting flows — which is possible given the uncertainty in the US macro environment — India will get its share.
Now an FPI does not have to sell India to buy China. India will not get a disproportionate share of the EM flows but the selling intensity can decline.
We look much better than we did a year and a half ago. Valuations were expensive. Are we at absolute rock-bottom valuations where one should put 100% into equities? I would not say that.
But we are much better positioned than we were in July 2024. A lot of thematic froth in manufacturing, defense, capital goods has gone away.
Q) There are 2 precious metals which have not lost their sheen even in 2026 – Gold & Silver. We have seen some volatility – how should one play this theme?
A) While gold and silver remain important, focusing only on these two commodities may be limiting. The world today is seeing geopolitical tensions and supply disruptions that impact a much wider set of commodities.
Commodity price movements are no longer restricted to gold, silver, or crude oil, but are extending into metals and other commodities as well. Investors should therefore look beyond just gold and silver and consider a broader range of commodities when approaching this theme.
Q) The December quarter earnings are underway – what is your take on the earnings which have so far?
A) The growth has just started in different pockets and the earnings season has been either in line or better than expectations including in challenged sectors like IT services. We have not witnessed any downward earnings revision as a result so far.
GST cuts have been structurally positive but the demand revival will probably come by fiscal 2027 and not really this year. In the last quarter we saw the starting signs of GST cuts working in the insurance and auto sector.
Last year, Nifty 50 earnings per share growth was in the 3% range. This year, it is likely to be in the 7–8% range. And next year (FY2027) the expectations are around 15%.
Q) Hiring has taken back seat in the Indian Technology sector. What is your take on the service space amid rupee depreciation, rise of AI and global slowdown?
A) IT downgrades have stopped, even though there are no strong upgrades. There will be no drag on corporate profitability for IT companies. That is a relief, though not a growth driver.
Q) How should one play the small & midcap theme?
A) Mid and small cap valuation premium (vs. Nifty50) has come down materially since mid-2024. This is providing opportunities to re-enter mid/small cap segments selectively.
(Disclaimer: Recommendations, suggestions, views, and opinions given by experts are their own. These do not represent the views of the Economic Times)
Business
Job Seekers Pay to Get Recruited, White-Collar Workers Switch to Trades and the Labor Market’s ‘Deep Freeze’ | Careers & Leadership for February 11
This is an edition of the WSJ Careers & Leadership newsletter, a weekly digest to help you get ahead and stay informed about careers, business, management and leadership. If you’re not subscribed, sign up here.
In the Workplace
Job hunters are so desperate that they’re paying to get recruited. Landing a white-collar job is getting so tough that candidates—not companies—are paying recruiters to match them with positions, a reversal of the traditional model.
Copyright ©2026 Dow Jones & Company, Inc. All Rights Reserved. 87990cbe856818d5eddac44c7b1cdeb8
-

 Politics4 days ago
Politics4 days agoWhy Israel is blocking foreign journalists from entering
-

 Sports6 days ago
Sports6 days agoJD Vance booed as Team USA enters Winter Olympics opening ceremony
-
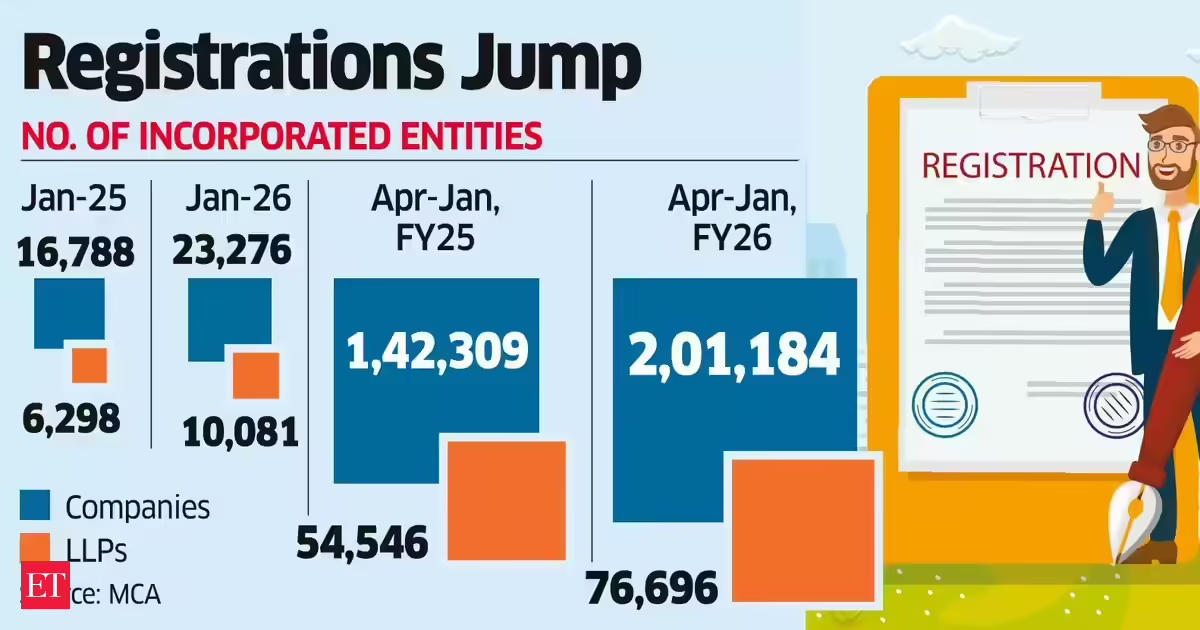
 Business4 days ago
Business4 days agoLLP registrations cross 10,000 mark for first time in Jan
-

 NewsBeat3 days ago
NewsBeat3 days agoMia Brookes misses out on Winter Olympics medal in snowboard big air
-
 Tech6 days ago
Tech6 days agoFirst multi-coronavirus vaccine enters human testing, built on UW Medicine technology
-

 Sports1 day ago
Sports1 day agoBig Tech enters cricket ecosystem as ICC partners Google ahead of T20 WC | T20 World Cup 2026
-

 Business4 days ago
Business4 days agoCostco introduces fresh batch of new bakery and frozen foods: report
-

 Tech2 days ago
Tech2 days agoSpaceX’s mighty Starship rocket enters final testing for 12th flight
-

 NewsBeat4 days ago
NewsBeat4 days agoWinter Olympics 2026: Team GB’s Mia Brookes through to snowboard big air final, and curling pair beat Italy
-

 Sports4 days ago
Sports4 days agoBenjamin Karl strips clothes celebrating snowboard gold medal at Olympics
-
Sports6 days ago
Former Viking Enters Hall of Fame
-

 Politics5 days ago
Politics5 days agoThe Health Dangers Of Browning Your Food
-
Sports7 days ago
New and Huge Defender Enter Vikings’ Mock Draft Orbit
-

 Business5 days ago
Business5 days agoJulius Baer CEO calls for Swiss public register of rogue bankers to protect reputation
-

 NewsBeat7 days ago
NewsBeat7 days agoSavannah Guthrie’s mother’s blood was found on porch of home, police confirm as search enters sixth day: Live
-
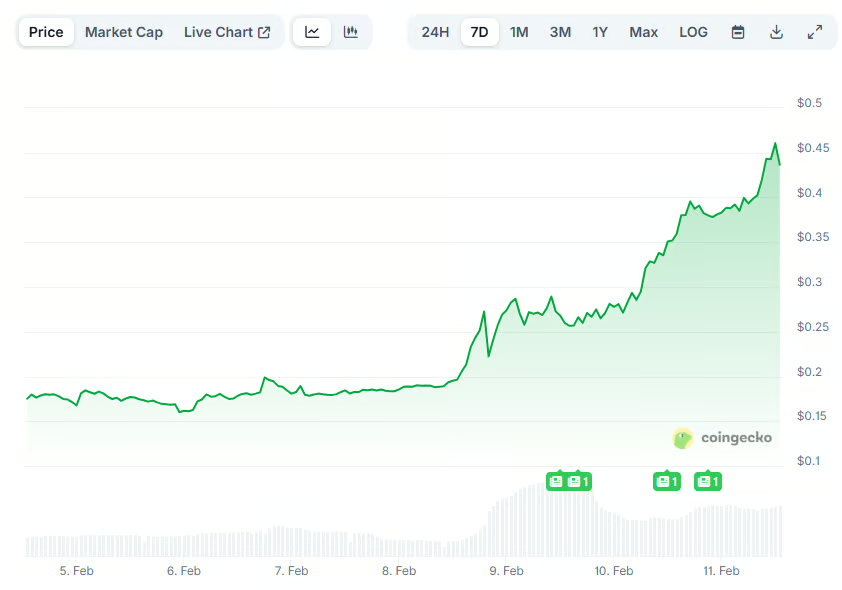
 Crypto World1 day ago
Crypto World1 day agoPippin (PIPPIN) Enters Crypto’s Top 100 Club After Soaring 30% in a Day: More Room for Growth?
-
 Video1 day ago
Video1 day agoPrepare: We Are Entering Phase 3 Of The Investing Cycle
-

 Crypto World3 days ago
Crypto World3 days agoBlockchain.com wins UK registration nearly four years after abandoning FCA process
-

 NewsBeat4 days ago
NewsBeat4 days agoResidents say city high street with ‘boarded up’ shops ‘could be better’
-
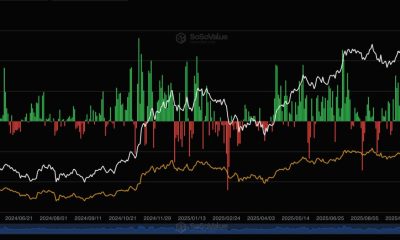
 Crypto World3 days ago
Crypto World3 days agoU.S. BTC ETFs register back-to-back inflows for first time in a month









